Name Used to Describe a Single Covalent Molecule
Although this form of covalent bond has a smaller density and is weaker than a double and triple bond it is the most stable. Simple molecules contain only a few atoms held together by covalent bonds.

Ib Chemistry Standard Level Notes Covalent Bonding
The name of the compound is dinitrogen tetroxide.

. A single covalent bond is called a single bond or single covalent bond. The element with the lower group number is written first in the name. Its first name is nitrogen and the second name is oxide.
Wiki User 2009-05-21 155042. List of Chemical Compounds and their uses Calcium Carbonate. Methanol is the antifreeze in automobile windshield washer fluids and it is also used as an efficient fuel for racing cars most notably in the Indianapolis 500.
Simple molecules contain only a few atoms held together by covalent bonds. It is customary to prefix the name of one atom of the second element with mono-. Carbon is able to form four single covalent bonds with other atoms and thus there are many carbon based molecules that use single.
When naming molecular compounds prefixes are used to dictate the number of a given element present in the compound. Single atoms of elements are not molecules. In a covalent compound of this type wed use the name of the first element then mono name of second element ide.
How do you name covalent molecular compounds. Since theres no number after the element you would write it simply as carbon. The prefix mono is never used for naming the first element of a compound.
The two atoms have similar electronegativity and The Electrons are shared equally between the two atoms. Lesson Summary A chemical bond is an attraction between two atoms that holds. Remember its only the final o or a.
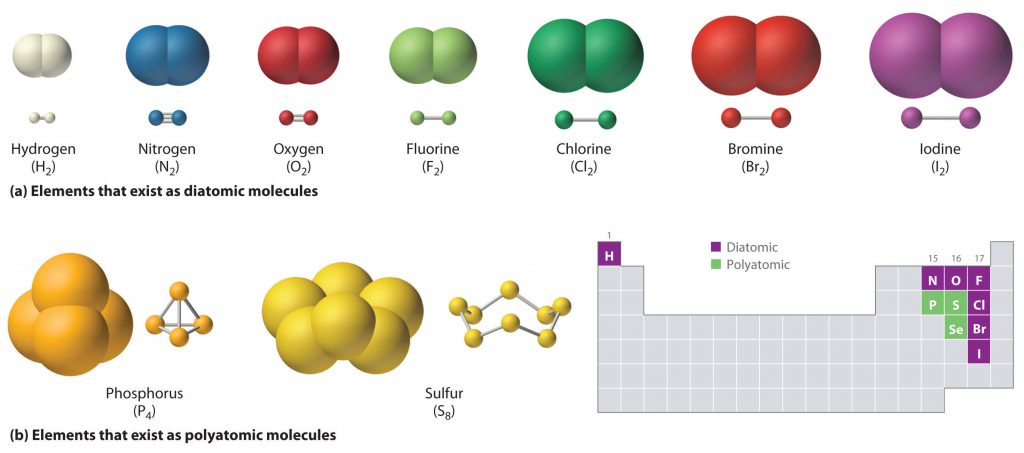
When oxygen bonds to itself eg O 2 O 3 or to another element eg carbon dioxide or CO 2 molecules are formed. What are the 10 compounds. Each alkane has a.
A single oxygen O is not a molecule. In structural formulas a single bond is represented by a line between bonded atoms. The most widely accepted format for nomenclature has been established by IUPAC.
Select all true statements about nonpolar covalent bond from the list below. Simple molecular compounds with common names. For example CO is named carbon monoxide rather than carbon oxide.
SF 6 Sulfur Hexaflouride. Some hydrocarbons have only single bonds and appear as a chain which can be a straight chain or can have branches of carbon atoms also bonded to hydrogen atoms. The compound is covalent since both nitrogen and oxygen are nonmetals.
What Is Not a Molecule. An example is carbon dioxide CO2 the molecules of which contain one atom of. Methane is the simplest organic compound.
H2 Hydrogen gas has one single covalent bond in between the two hydrogen atoms. For example if you were working with the formula CF₄ the first element is C which is carbon. H 2 O 2.
For example for CO the name will be carbon monoxide and the final o of mono is dropped. Today scientists often refer to chemicals by their common names. We have already encountered these compounds but we list them here explicitly.
How to Name Binary Acids and Oxyacids in General. It is represented by one dash -. These hydrocarbons are called alkanes saturated hydrocarbons A hydrocarbon with only single covalent bonds and existing as a chain of carbon atoms also bonded to hydrogen atoms.
Only use numerical prefixes for the first element when the molecule contains more than one of that atom. Sigma bond in the hydrogen molecule. This requires you to know the standard greek prefixes for numbers.
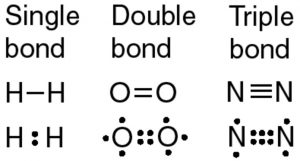
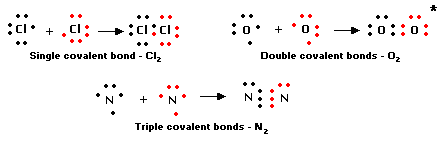
For instance the diatomic hydrogen molecule H 2 can be written as HH to indicate the single covalent bond between the two hydrogen atoms. Examples of Covalent Compound Names. Single Covalent Bond Double Covalent Bond Triple Covalent Bond.
Mono- indicates one di- indicates two tri- is three tetra- is four penta- is five and hexa- is six hepta- is seven octo- is eight nona- is nine and deca is ten. Is n2o4 a covalent compound. Each chemical name should refer to a single substance.
The simplest alcohol CH 3 OH is called either methanol its systematic name or methyl alcohol its common name see Figure 24 Different Ways of Representing the Structure of a Molecule. How to write the molecular formula of a covalent molecule given the long-form name. For example a molecule of chlorine trifluoride ClF 3 contains 1 atom of chlorine and 3 atoms of fluorine.
The element with the higher group number is written second in the name. A single bond is formed when only one pair of the electron is shared between the two participating atoms. CO 2 Carbon Dioxide N 2 O 4 Dinitrogen Tetroxide.
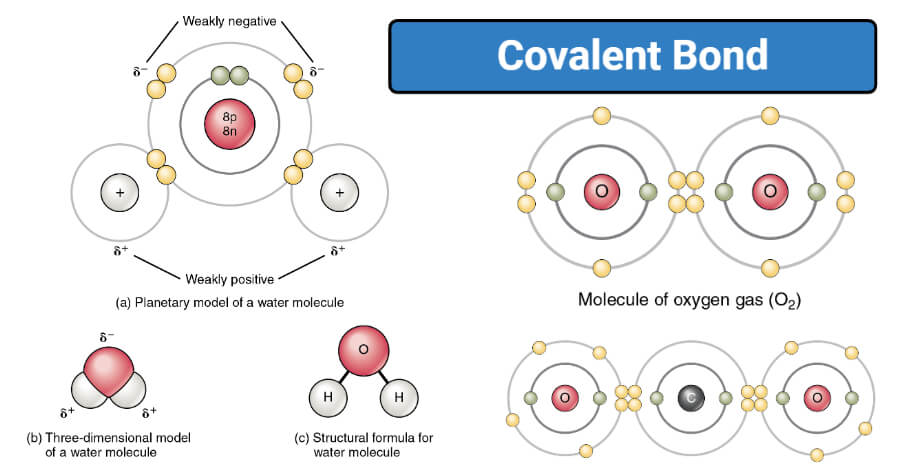
Higher intensity of the red color indicates a greater probability of the bonding electrons being localized between the nuclei. N2O is the chemical formula of a covalent compound used in the production of whipping cream. The final o or a of a prefix is often dropped when the element begins with a vowel.
For example water is not often called dihydrogen oxide. Some other molecules also have the single covalent bonds as HFHClHBr and HI. However it is important to be able to recognize and name all chemicals in a standardized way.
How to write the long-form name of a covalent molecule given the molecular formula. Rules for Binary Covalent Compounds. A single covalent bond is one in which two electrons are shared by two atoms in which one electron comes from each atom.
A binary covalent compound is composed of two different nonmetal elements. An example is carbon dioxide CO2 the molecules of which contain one atom of. The chemical formulas for the common elements water ammonia methane and ozone.
The following diagram represents the structural formula of pentane. To name covalent compounds youll need to figure out how many atoms of each element the compound contains. For some simple covalent compounds we use common names rather than systematic names.
In a water molecule H2O the ___ atom has a partial negative charge and the ___ atom has a partial positive charge. Types of covalent bonds are. Methane which is a carbon atom with single covalent bonds to four hydrogen atoms is the simplest carbon compound.

Single Covalent Bond Definition And Examples

Single And Multiple Covalent Bonds Article Khan Academy

Single And Multiple Covalent Bonds Article Khan Academy
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
Covalent Bond Definition Properties Types Examples

Covalent Bond Biology Dictionary

Single Covalent Bond Covalent Bonding Teaching Chemistry Bond
Covalent Bond Chemical Bonding And Molecular Structure Chemistry Class 11

Ib Chemistry Standard Level Notes Covalent Bonding

Single Covalent Bond Definition And Examples

Single Covalent Bond Definition And Examples

Covalent Compounds Manoa Hawaii Edu Exploringourfluidearth

Covalent Bond Definition Types And Examples
Chemistry I Atoms And Molecules

Ib Chemistry Standard Level Notes Covalent Bonding
How To Distinguish Between Single Double And Triple Covalent Bonds Quora
Ch103 Chapter 5 Covalent Bonds And Introduction To Organic Molecules Chemistry

Comments
Post a Comment